Does the
Valence State of an Ion Affect its Diffusivity? - Part I: Oxygen Activity
Dependence of the Diffusion of Iron in Alumina-Doped MgO
E. Chen, T.-L. Tsai and R. Dieckmann
Department of Materials Science and Engineering,
Cornell University, Bard Hall, Ithaca, NY 14853-1501, U.S.A.
Abstract
To investigate whether a change in the valence state of tracer ions
affects their diffusivity or not, the iron tracer diffusion in Al2O3-doped
MgO, in which 0.5 % of the cations were Al3+ ions, has been studied
experimentally. Samples were prepared from high purity aluminum and magnesium
nitrates using a chemical solution method and from powders of high purity Al2O3
and MgO. Because the concentration of the Al3+ dopant ions present
in the samples was much larger than that of all other impurities, the
concentration of the majority point defects, cation vacancies, was determined
by the Al3+ concentration. Therefore, when changing the oxygen
activity, the diffusivity of iron tracer ions can only be altered by changes in
their valence state. Measurements of iron tracer diffusion coefficients were
performed as a function of the oxygen activity at 1100 and 1200 °C. The
experimental results indicate that the mean diffusivity of iron ions in Al2O3-doped
MgO increases with increasing oxygen activity at both temperatures, suggesting
that Fe3+ ions diffuse in Al2O3-doped MgO
faster than Fe2+ ions.
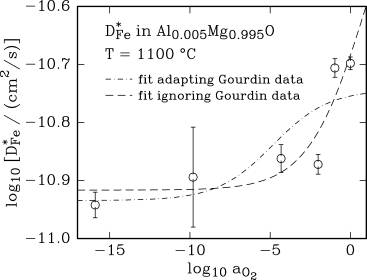
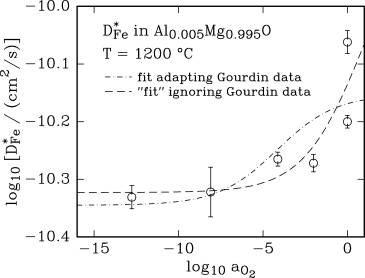
Oxygen activity dependence of the iron tracer diffusion
coefficient, D*Fe, at 1100 and
1200 °C.
Solid State Sciences, 10 (6) [2008]
735-745.